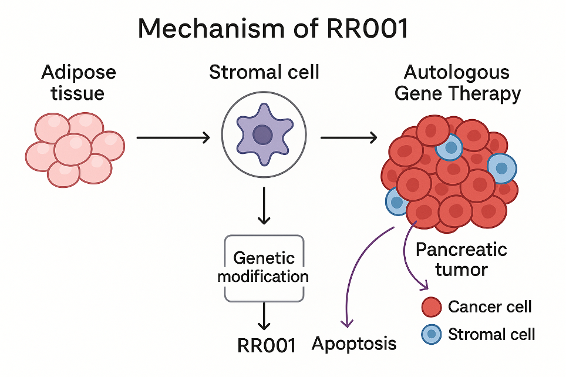
RR001 is a genetically modified cell to produce a multimeric TRAIL protein variant that kills both tumoral cells and stromal cells (namely cancer associated fibroblasts – CAF) which are supporting tumor growth. RR001 is an autologous gene therapy—meaning it is made from a patient’s own cells—that has been genetically modified to help treat pancreatic cancer, specifically ductal adenocarcinoma.
What is RR001?
RR001 is an autologous gene therapy based on healthy stromal cells derived from the patient’s adipose tissue. These cells are modified in the lab to produce a modified version of the TRAIL protein (TNF-related apoptosis-inducing ligand).
How does RR001 act?
RR001 targets cancer cells and their tumor microenvironment, specifically the stromal cells (namely cancer associated fibroblasts – CAF) that surround and support the growth of pancreatic tumors. These cells additionally form a dense barrier that protects the tumor and limits the effectiveness of conventional therapies.
Once administered back into the patient, RR001 relocate to the tumor site and release the TRAIL protein, which binds to death receptors on nearby tumor and cancer associated fibroblasts. This triggers apoptosis (programmed cell death) in both those cells.
In the SNIPER trial, RR001 is being combined with chemotherapy (nab-paclitaxel and gemcitabine) in patients with locally advanced, non-metastatic pancreatic cancer.
The goals of RR001 in this setting are:
In the SNIPER trial, RR001 is being combined with chemotherapy (nab-paclitaxel and gemcitabine) in patients with locally advanced, non-metastatic pancreatic cancer.
The goals of RR001 in this setting are:
Details of the SNIPER study
In English, sniper means “sharpshooter”—an apt name for a clinical trial targeting pancreatic cancer with precision gene therapy. Ductal adenocarcinoma is the most common form of pancreatic cancer and is primarily treated with surgery. However, only a minority of patients are eligible for surgery at diagnosis, and 5-year survival remains low. Preoperative neoadjuvant chemotherapy improves survival, but only a quarter of patients make it to surgery.
SNIPER is a Phase I/IIa, open-label clinical trial (ClinicalTrials.gov: NCT06861452) testing a combination of chemotherapy and gene therapy in the preoperative setting. The gene therapy, RR001, uses autologous adipose-derived cells genetically modified to produce a TRAIL protein variant that kills stromal cells supporting tumor growth. Developed by Dominici’s group at the University of Modena and Reggio Emilia, in collaboration with EIR Biotherapies and Evotec Modena, RR001 was validated in 3D and animal models, showing the ability to weaken tumors by disrupting their microenvironment.
Researchers then combined RR001 with standard drugs nab-paclitaxel and gemcitabine, noting enhanced tumor and stroma damage. SNIPER now aims to determine the safety, feasibility, and optimal dose of RR001 plus chemotherapy. Conducted at AOU Modena by Dr. Annalisa Fontana and Dr. Andrea Spallanzani, as PI and CO-PI the trial will enroll nine patients with locally advanced, non-metastatic pancreatic cancer.
The goal is to increase the number of patients eligible for surgery and reduce time to resection, potentially improving survival. While many immunotherapies have changed the landscape for other GI cancers, pancreatic adenocarcinoma remains challenging. Even when surgery isn’t possible, the SNIPER protocol may enhance chemotherapy response and prolong survival—a major step forward in advanced cancer therapy.
EIR Biotherapies
info@eirbiotherapies.com